Synthesis and biological evaluation of cationic fullerene quinazolinone conjugates and their binding mode with modeled Mycobacterium tuberculosis hypoxanthine-guanine phosphoribosyltransferase enzyme.
Patel MB, Kumar SP, Valand NN, Jasrai YT, Menon SK.
Department of Chemistry, University School of Sciences, Gujarat University, Ahmedabad, Gujarat, 380009, India. Department of Bioinformatics, Applied Botany Centre, University School of Sciences, Gujarat University, Ahmedabad, Gujarat, 380009, India.

Department of Chemistry, University School of Sciences, Gujarat University, Ahmedabad, Gujarat, 380009, India. Department of Bioinformatics, Applied Botany Centre, University School of Sciences, Gujarat University, Ahmedabad, Gujarat, 380009, India.

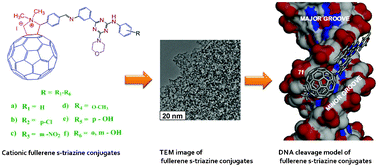
The present work reports a series of novel cationic fullerene derivatives bearing a substituted-quinazolinone moiety as a side arm. Fullerene-quinazolinone conjugates synthesized using the 1,3-dipolar cycloaddition reaction of C60 with azomethine ylides generated from the corresponding Schiff bases of substituted quinazolinone were characterized by elemental analysis, FT-IR, 1H NMR, 13C NMR and ESI-MS and screened for their antibacterial activity against Mycobacterium tuberculosis (H 37 Rv strain). All the compounds exhibited significant activity with the most effective having MIC in the range of 1.562-3.125 μg/mL. Compound 9f exhibited good biological activity compared to standard drugs. We developed a computational strategy based on the modeled M. tuberculosis hypoxanthine-guanine phosphoribosyltransferase (HGPRT) using homology modeling techniques and studied its binding pattern with synthesized fullerene derivatives. We then explored the surface geometry of the protein to place the cage adjacent to the active site while optimizing its quinazolinone side arm to establish H bonding with active site residues.
Access full text here: